


Next: Quality of the aerogels
Up: Detector Design
Previous: Detector Design
Contents
Since aerogels having the required low refractive indices were not
commercially available, we decided to produce aerogels by our own
efforts. We adopted a new production method for the preparation of
aerogels, in which methylalkoxide oligomer is used as a
precursor [45]. This oligomer is hydrolyzed and
polymerized in a basic catalyst (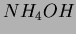 ) in a solution of methyl or
ethyl alcohol. The average size of alcogels is
) in a solution of methyl or
ethyl alcohol. The average size of alcogels is
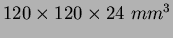 and they are formed in aluminum molds coated with a thin
PTFE film. The gelation time ranges from a few minutes to 10 min
depending on densities.
and they are formed in aluminum molds coated with a thin
PTFE film. The gelation time ranges from a few minutes to 10 min
depending on densities.
Silica aerogels have been used in several experiments, but their
transparencies became worse within a few years of use, which was our
great worry to adopt an aerogel Cerenkov counter as a particle
identification device. This phenomenon may be attributed to the
hydrophilic property of silica aerogels. In order to prevent such
effects, we have made our silica aerogels highly hydrophobic by
changing the surface hydroxyl groups into trimethylsilyl
groups [46]. This modification is applied before the
drying process. As a result of this treatment, our silica aerogels
remain transparent even four years after they were produced.
After three weeks of aging including the surface modification, the
alcogels were dried by a super critical drying method of 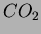 . This
drying process took 48 h. The volume of extractor is 140
. This
drying process took 48 h. The volume of extractor is 140 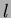 and we
could produce about 38
and we
could produce about 38 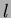 of silica aerogel in one batch. After
seven months of operation (two batches/week), we produced about
2
of silica aerogel in one batch. After
seven months of operation (two batches/week), we produced about
2 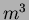 of silica aerogel. Details of the production method can be
found in [45,47].
of silica aerogel. Details of the production method can be
found in [45,47].



Next: Quality of the aerogels
Up: Detector Design
Previous: Detector Design
Contents
Samo Stanic
2001-06-02