 |
 |
||||||||||
X-RAY ABSORPTION SPECTROSCOPY AND RELATED TECHNIQUES
Related publications:: A. Kodre, I. Arčon, and J. Padeľnik Gomilšek, Acta Chim. Slov. 51, (2004) 1-10
Abstract
In the extensive field of x-ray diagnostic techniques, nowadays mostly implemented on synchrotron x-ray sources, the x-ray absorption methods offer a relatively simple tool for structural analysis of materials. The advantage of the methods is the sensitivity to chemical species and the insensitivity to the long-range order. An overview of various detection techniques is given together with the discussion of accuracy of the method with regard to the spatial resolution and the possible contamination by intra-atomic contributions, the multielectron photoexcitations.
Introduction
The considerable diagnostic power of x-rays is based on a coincidence of two fortuitous properties: the wavelength of the x-ray light is of the same order of magnitude as interatomic distances, and the energy of the x-ray photons is of the order of binding energies of the most tightly bound electrons.The first property provides for rich and meaningful interference patterns of the light in a beam passing a layer of a well-ordered material.From this the positions of the constituent atoms in the basic unit of a periodic structure can be deduced, leading to various techniques in XRD ( = X-Ray Diffraction) developed for either large monocrystals or microcrystalline powders [1,2]. Even the nano- and mesoscale inhomogeneities of the materials are discernible in the interference pattern close to the direct beam (SAXS – Small Angle X-ray Scattering [2].
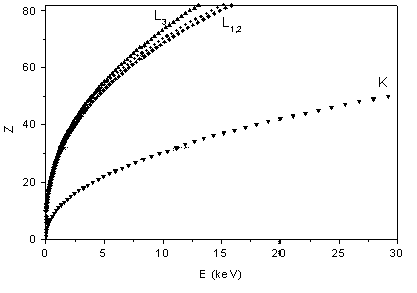 |
The second property of x-rays opens a way for a fast and simple elemental analysis: the binding energies of the core electrons grow monotonically with atomic number (Moseley law), unaware of chemical periodicity (Fig. 1). Thus, constituent elemental species of a sample can be identified simply from the energies of absorption edges in its x-ray absorption spectrum or from the energies of the characteristic x-ray lines in its fluorescent radiation. This is the basis of modern XRF ( = X-Ray Fluorescence) analytic techniques which can even be exploited for a fast quantitative analysis [3].
|
| Fig. 1. The relation between the atomic number Z of an element and the energy of its K and L x-ray absorption edges (Moseley law). |
The simple dependence of the characteristic energies on the atomic number is also exploited in X-ray absorption spectroscopy (XAS) [4,5,6]. The basic experiment is very simple: a thin homogeneous sample of the investigated material is prepared, and the intensities of the incident and the transmitted x-ray beam are recorded in the stepwise progression of the incident photon energy (Fig. 2).
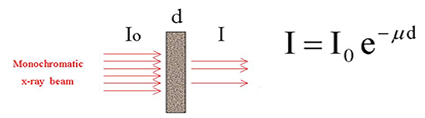 |
| Fig. 2a. A schematic representation of x-ray absorption spectroscopic measurements in trasmission mode. |
 |
|
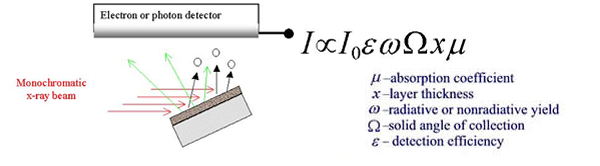
Fig.
2b. A schematic representation of x-ray absorption
spectroscopic measurements in fluorescence and electron
detection
mode. |
|
The scheme of a typical absorption beamline at a synchrotron radiation
source is shown in Fig. 3. Ionisation cells monitor the intensity
of incident (Io) and transmitted (I1) monochromatic photon beam
through the sample. With the well-known exponential attenuation
of x-rays in a homogeneous medium, the absorption coefficient |
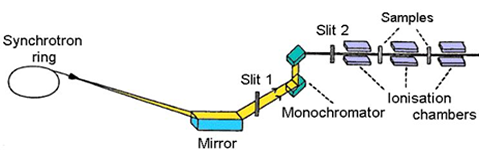 |
Fig.
3. Schematic view of E4 x-ray beamline at Hamburger
Synchrotron Radiation Laboratory (HASYLAB) at DESY in Hamburg. |
| |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| E-mail:iztok.arcon@p-ng.si Last change: 31-Mar-2004 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||