 |
 |
||||||||||
EXAFS analysis of bimetalic Pd/Cu catalysts
Related
publications:
1) J.
Batista, A. Pintar, J. Pade¾nik Gomil¹ek, A. Kodre, F. Bornette,
A Gen. (2001), vol. 217 ,(2001) no. 1/2, 55-68.
2)Alojz Kodre,
Iztok Arcon, Jurka Batista, Albin Pintar, J. Synch. Rad. 6 (1999) 458-459
Abstract
The structure of Pd/Cu catalysts on g-alumina for nitrate/nitrite
ion removal from drinking water is investigated. EXAFS spectra at
the K edge of both elements show that small clusters with the basic
crystal structure of bulk palladium metal are formed. The size of
the clusters is estimated from the range of photoelectron scattering
paths, and from the average number of first neighbors. Both estimates
indicate clusters of about 12 atoms, ie two neighbor shells.
Introduction
Palladium metal is one of the most promising catalysts for removing
nitrate and nitrite ion from drinking water (Hörold
et al., 1993, Pintar et al., 1996). For kinetic studies, monometallic Pd and bimetallic
Pd-Cu catalysts were prepared by impregnation of g-Al2O3 powder with
Pd and Cu nitrate salts (5 and 1.5 at.% respectively) and subsequent
calcination/reduction in hydrogen atmosphere.
EXAFS spectra of the samples at the K edge of Pd and Cu were measured
at the ROEMO II and EXAFS II beamlines of HASYLAB at DESY, Hamburg,
with resolution of 3 eV and 1.5 eV, respectively. Higher harmonics
of the beam were eliminated by a slight detuning of the monochromator
crystals. Due to low metal content and low density the optimum
absorption samples were prepared by pressing the powder into
3 mm layers between
the windows of a liquid-absorption cell.
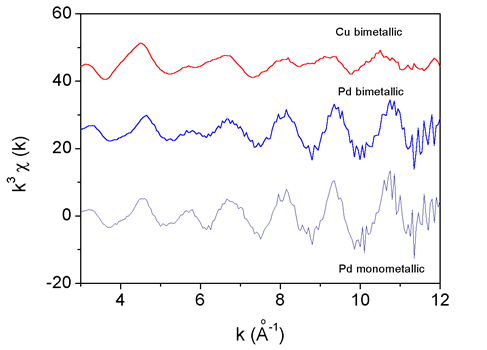 |
Fig. 1. Pd and Cu K edge EXAFS spectra of the monometallic and bimetallic catalysts. |
The measured EXAFS spectra (Fig. 1) were analysed by the UWXAFS
code (Stern et al., 1995, Rehr et al., 1992) in the k-range
3.5 - 12 C-1,
using k2 weight and Hanning window. The shape of the Pd K absorption
edge, identical with that of bulk Pd, suggests a model based
on the fcc crystal structure of Pd metal. When applied to
the spectrum of
the monometallic catalyst, excellent fit for the FT region
from 2°C to 5.5°C, is found using the 8 shortest scattering
paths
of the lattice.
For the lower part of the FT spectrum in the range 1 - 2°C,
a shell of oxygen atom neighbors at 1.9 C, presumably from
the
contact layer
with the substrate, is introduced into the model. The agreement
(Fig. 2) indicates that clusters with fcc structure of Pd
metal are formed.
An estimate of their size is given by the cutoff in the FT
spectrum at approx. 6.0°C. Specifically, the absence of a prominent
peak
at 7°C in FT spectra of bulk Pd provides a definite upper
limit. A better
estimate of the average cluster size is obtained from the number
of first neighbors (5.9) which is considerably lower than
the coordination
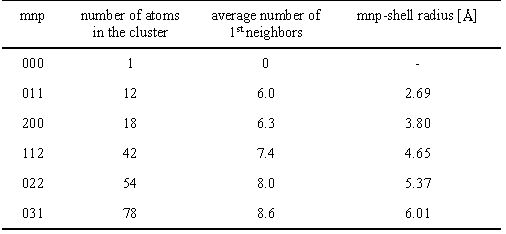
number 12 of an infinite lattice. A calculation (Table 1) shows
that the observed value is reached in a cluster of about
12 atoms in a tentative
shape of an octahedron with a diameter of 5.5°C formed by a
layer of nearest neighbors around a central atom.
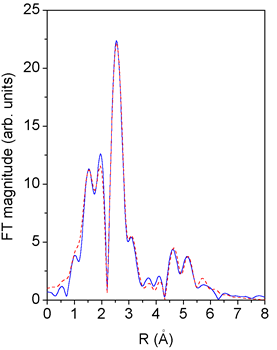 |
Fig. 2. Fourier transform magnitude of Pd K-edge EXAFS spectra measured on monometallic Pd catalysts: experiment - solid line, fit - dashed line. |
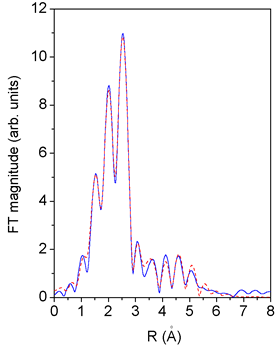 |
| Fig. 3. Fourier transform magnitude of Pd K-edge EXAFS spectra measured on bimetallic Pd-Cu catalysts: experiment - solid line, fit - dashed line. |
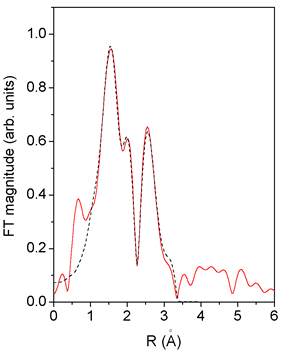 |
| Fig. 4. Fourier transform magnitude of Cu K-edge EXAFS spectra measured on bimetallic Pd-Cu catalysts: experiment - solid line, fit - dashed line. |
Since the Pd EXAFS spectrum of the bimetallic Pd-Cu catalyst retains the characteristic fcc shape, the same cluster model with addition of a shell of Cu atoms at the contact Pd-Cu distance (2.65°C) is applied. Again, the agreement is very good, as shown in Fig. 3. Apart from a slight but significant change in the Pd-Pd distance, the best fit values of the corresponding model parameters for the bimetallic and monometallic clusters are essentially the same (Table 2).
The FEFF model for the Cu EXAFS spectrum of the bimetallic sample
is constructed from the bimetallic Pd fcc model by substitution
of the
central atom, retaining the four shortest scattering paths to fit
the r-range 1.2°C - 3.5°C. The fit (Fig. 4) shows that the
Cu neighborhood
is less populated by metal atoms than that of Pd (Table 3). Together
with the stronger Cu-O correlation this indicates that the Cu atoms
are attached to the Pd cluster. The description of Pd-Cu bonds, as
seen from both atoms, agrees well in distance parameter, and satisfactorily
in the coordination number. The s2 parameters, however, cannot be
reconciled.
Support by the Ministry of Science and Technology of the Republic
of Slovenia, and Internationales Bhro (Germany) is acknowledged.
M. Tischer
of HASYLAB provided expert advice on beamline operation.
Table 1. Average number of first Pd neighbors in clusters with maximum mnp site indices. mnp number of atoms in the cluster average number of 1st neighbors mnp-shell radius [C] |
 |
Table
2. Model parameters of the EXAFS at the Pd K edge. |
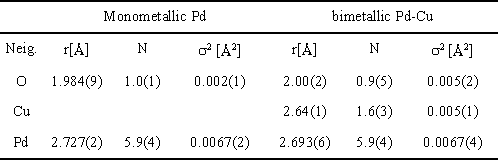 |
Table 3. Model parameters of the EXAFS at the Cu K edge for Pd-Cu sample. |
 |
References
Hörold, S., Tacke, T. & Vorlop, K. D. (1993). Environ. Tech.
14, 931-939.
Pintar, A., Batista, J., Levec, J. & Kajiuchi, T. (1996). Appl.
Catal. B:Environmental 11, 81-98.
Rehr, J., Albers, R. C. & Zabinsky, S. I. (1992). Phys. Rev.
Lett. 69, 3397–3400.
Stern, E. A, Newville, M., Ravel, B., Yacoby, Y. & Haskel, D. (1995).
Physica B 208&209, 117-120.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
E-mail:iztok.arcon@p-ng.si Last change: 02-Jun-2006 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||